
Reimbursement process: how stakeholders organise the series of steps to take
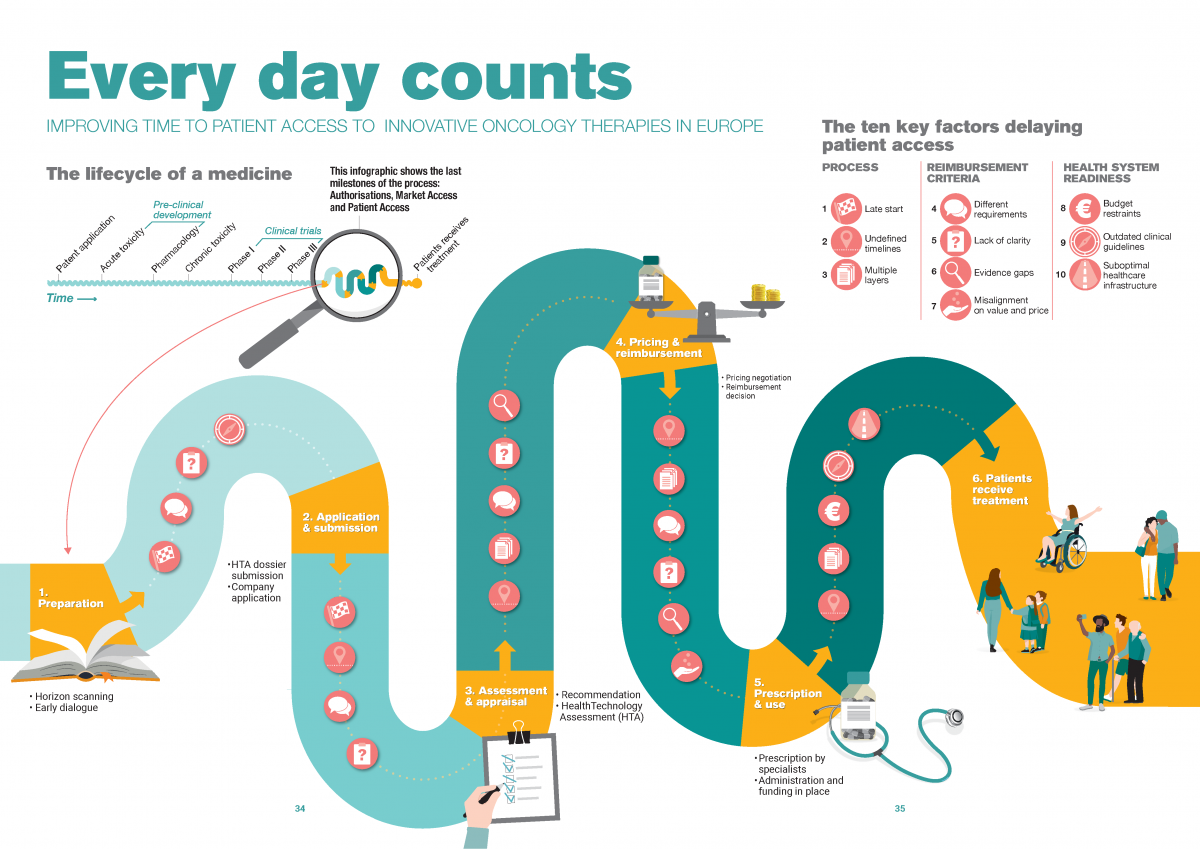
1. Late start of application and submission
Compared to European marketing authorization, the national reimbursement process may start late for two reasons: due to country regulations on the start of the process or due to manufacturer submission timelines under the influence of external reference pricing.
2. Lack of adherence to maximum timelines
Despite the maximum of 180 days set by the EU Transparency Directive, most European countries do not adhere to a clear set of rules around the timelines for decision-making on national pricing and reimbursement. Even when countries have such rules in place, compliance can be challenging, for example due to a scarcity of human resources within both HTA bodies and pharmaceutical companies.
3. Multiple layers of decision-making
After a positive decision on national reimbursement, some countries add one or more extra layers by allowing regional decision-makers or hospitals to individually decide on reimbursement or budget allocation. As a result, we see duplication, in-country disparities and prolonged waiting times for cancer patients.
Reimbursement criteria: what information stakeholders use to define value
4. Different evidence requirements across Europe
Evidence requirements vary greatly across healthcare assessment agencies, it’s near impossible for pharmaceuticals to meet all requirements with one global dataset. As a result, pharmaceutical companies may need to collect additional country-specific data, leading to considerable delays.
5. Lack of clarity of national requirements
Even within countries, criteria and requirements for clinical and cost-effectiveness assessments are not always clearly defined and may vary on a case-to-case basis. Although case-dependency allows for a tailor-made assessment, it results in an unpredictable evaluation and prolongs alignment.
> Read our blog on national reimbursement requirements across Europe
6. Evidence gaps
Meeting healthcare assessment agencies’ evidence requirements is increasingly difficult given the characteristics of today’s oncology therapies , leading to evidence gaps and uncertainty about the value of the therapy.
7. Misalignment on value and price
Uncertainty about the value of the therapy leads to misalignment on value and price and long negotiations between national decision-makers and pharmaceutical companies. Especially in the absence of black-and-white assessment criteria and ways to handle evidence gaps.
Health system readiness: to what extent stakeholders integrate a new therapy in clinical practice
8. Insufficient budget to implement decisions
After a positive reimbursement has been made for a treatment, a country needs to ensure enough budget is available to implement this decision and to fund the medicine for the entire financial year. Delays in ensuring implementation budget or budget depletion place a negative pressure on the prescription and use of the new medication.
9. Low frequency of clinical guideline updates
Even for the five main cancer types in Europe, in many countries clinical guidelines do not include the most recent therapeutic innovations, resulting in a lack of clarity on when and where to use the therapy in the treatment pathway. As a result, healthcare providers may be hesitant to prescribe and use a new treatment.
10. Suboptimal healthcare infrastructure
As basic conditions, patients need to have access to high quality health facilities, diagnostic centres and health personnel. More specifically, the oncology care pathway should facilitate the optimal use of innovative therapies through early diagnosis and access to the latest therapies. However, healthcare systems in general and oncology pathways in particular are not optimally organised. For that reason, many European countries are facing challenges in absorbing and using a new treatment.
SUBOPTIMAL HEALTHCARE INFRASTRUCTURE DURING THE COVID-19 PANDEMIC.
For oncology care, the need for a resilient healthcare infrastructure is especially urgent and unmet during today’s COVID-19 pandemic. The early weeks of the pandemic caused a 46% decrease in cancer diagnoses and a 44% of breast cancer survivors experienced a delay in care in the United States (Papautsky & Hamlish, 2020; Kaufman et al, 2020). Similar upsetting findings are reported in Europe (Dinmohamed et al., 2020; Joode et al., 2020). These disruptions demonstrate to what extent healthcare infrastructures lack resilience for maintaining high-quality, evidence-based continuity of cancer care. This includes workforce and service capacity needs, plus robust referral and diagnostic services.
Call for joint problem solving
To reduce the immense inequalities in patient access between European countries, pharmaceutical companies, policy makers, HTA bodies, payers, healthcare professionals and payers needs to find a common understanding of the problem and a common perspective on solutions. In today’s system, none of the stakeholders involved can solve today’s challenges single-handedly. We need a collaborative approach now. As for patients, every day counts.
LET’S DISCUSS
Inspired to share your thoughts? Or would you like to learn more on how to accelerate patient access? We would be delighted to hear from you. Please feel invited to contact Bas Amesz.
DO YOU WANT TO READ MORE?
Please also be invited to read our others articles in this “Every day counts” series:



