Our impact analysis applied three hypothetical reimbursement scenarios to real life cases from the past, using two case study therapies in six countries (Sweden, the Netherlands, England, Italy, Poland, Portugal). A three-step health economic model was developed to calculate: the difference in days between the actual reimbursement timelines and the optimised scenario, the number of additional patients that could have been treated and the health gains these treated patients would achieve. Let’s dive in.
Case study therapies: midostaurin and pertuzumab
Aiming to calculate the impact of earlier reimbursement, we included midostaurin for patients with a rare blood cancer and pertuzumab for pre-surgery early breast cancer. The data on European marketing authorisation, the date of Market Access, the number of new patients on the therapy (uptake) per month and health impact were retrieved respectively from EMA, the manufacturer and national or EUnetHTA assessment reports. Information from assessment reports was retrieved from public sources where possible and, when needed, complemented by manufacturers.
Impact of three scenarios
Using this input, for each therapy and country, the impact of three scenarios was calculated, ensuring the time to reimbursement (start of uptake) changed but the number of new patients per month remained equal.
- Highly ambitious scenario: reimbursement at the time of the European marketing authorisation; the time to Market Access is as short as possible.
- Best practice scenario: all countries ensure Market Access as fast as the fastest country.
- Basic scenario: reimbursement no later than 180 days after the European marketing authorisation, in conformity with the EC Transparency Directive.
Immense health gains
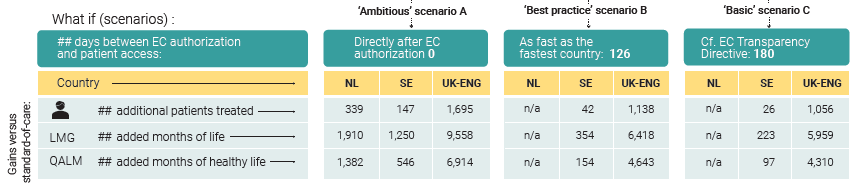
Even in the basic scenario, patients would benefit immensely from accelerating the decision-making process.
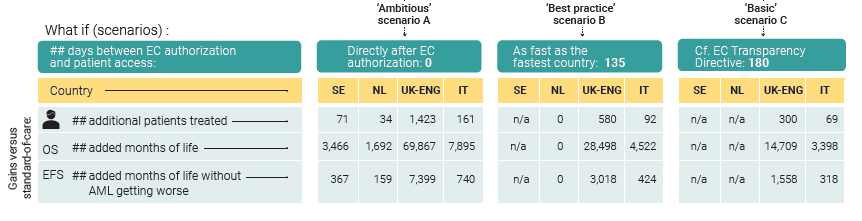


- If midostaurin had been reimbursed in England and Italy as fast as agreed in the EC Transparency Directive (within 180 days, a condition fulfilled by the Netherlands and Sweden) 369 more patients with AML could have been treated. They could have lived altogether an additional 18.107 months. This would have corresponded to 1.876 months without the disease getting worse.
- If pertuzumab had been reimbursed in Sweden and England as fast as agreed in the EC Transparency Directive (a condition fulfilled by the Netherlands): 1.083 more patients with early breast cancer could have been treated. They could have lived altogether an additional 6.181 months. This would have corresponded to 4.408 months when adjusted for the quality of life.
Imagine the impact of a more ambitious scenario, where all countries ensure reimbursement as fast as the fastest country or even as soon as a new therapy receives European marketing authorization. These outcomes show: for cancer patients, every single day counts. Let the outcomes serve as a reminder of the urgency of addressing delays wherever we can. As we’re dealing with a systemic issue, it’s our shared responsibility to step over our own shadow. We owe that much to cancer patients.
Let’s discuss
Inspired to share your thoughts? Or would you like to learn more on how to accelerate patient access? We would be delighted to hear from you. Please feel invited to contact Bas Amesz.
DO YOU WANT TO READ MORE?
Please also be invited to read our others articles in this “Every day counts” series:
- White paper release: Every day counts
- Blog: Bringing stakeholders together to improve patient access to oncology therapies in Europe
- Blog: European access discussions shouldn’t stop at reimbursement
- Blog: Deep dive: The Patient Access Indicator
- Blog: The 10 key factors delaying patient access across Europe



