Many may know that this process officially takes 67 days. But what actually happens during this process? And how can we streamline it and help ensure that vital new treatments are available sooner – given that every 50 days spent waiting for approval costs more than 20,000 years of life across Europe?
Vintura believes that demystifying every process and stage in a drug’s journey from R&D to approval, marketing and prescription is a vital step in promoting effective, cross-stakeholder discussions that can realize opportunities to reduce those timelines to increase patient value.
Our latest report in the series Every Day Counts can be downloaded here.
An administrative process of 67 calendar days
Before patients can access a new therapy, the European Commission (EC) must grant it a marketing authorization. This follows the CHMP decision, and the process officially takes 67 days.
The timeline is split into two phases: a linguistic phase, in which product information is translated into all 24 official EU languages, and a decision-making phase, in which the EC prepares its decision to grant or refuse a centralized marketing authorization (see below).
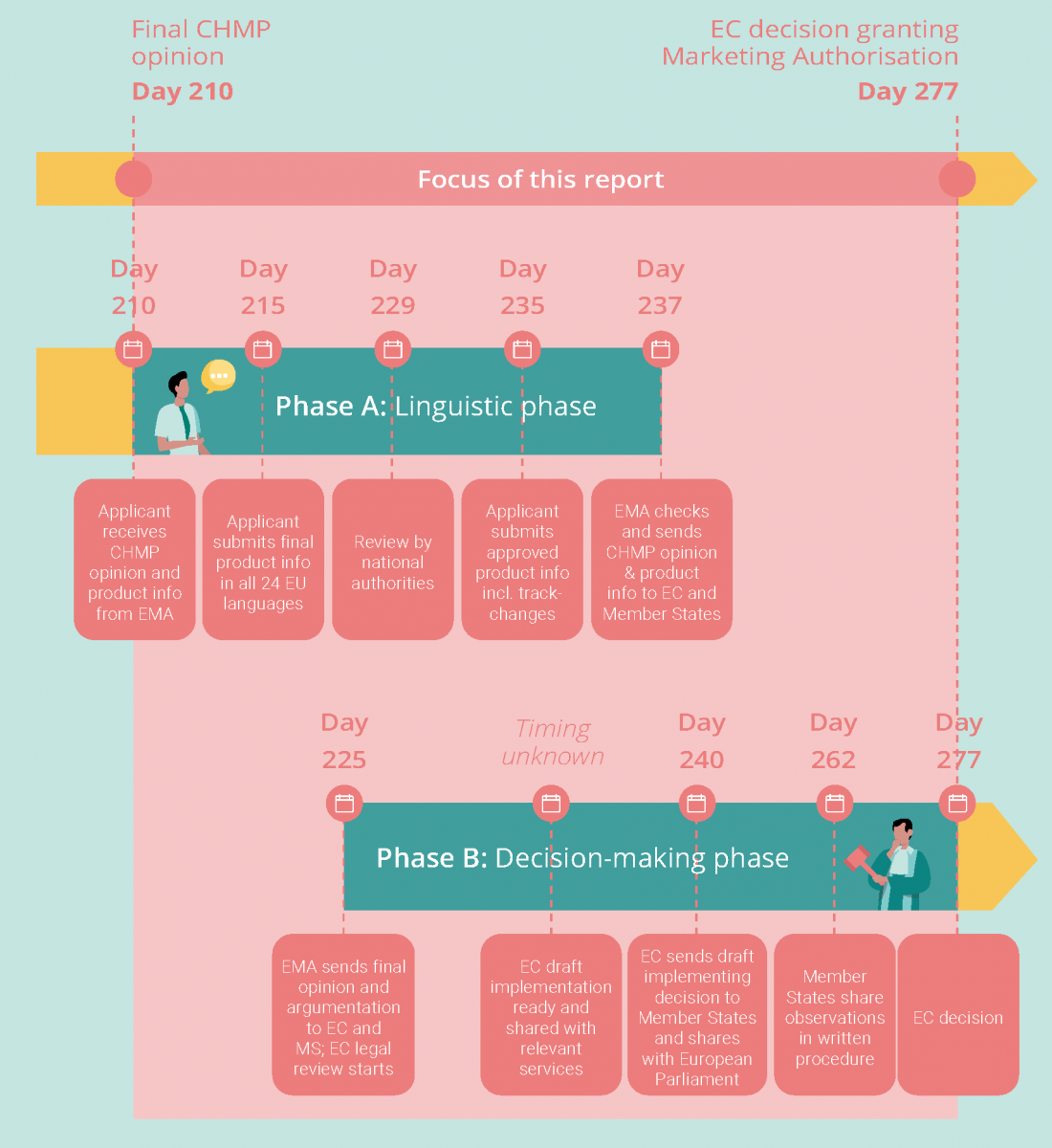
There are four key milestones in the linguistic phase, which together take an official 22 days:
- Day 215: Following the adoption of the final CHMP opinion, manufacturers have five calendar days to send the final product information, translated into all official EU languages (incl. Icelandic and Norwegian), to EMA. Product information includes a summary of the product’s characteristics, plus details of its active ingredients, supply and use, leaflet, and packaging.
- Day 229: Each translation is subject to linguistic review by Member States, for which they have 14 calendar days.
- Day 235: Upon receipt of Member States’ feedback, the manufacturer has six calendar days to send final translations to EMA, incorporating Member States’ comments in tracked changes.
- Day 237: EMA has two calendar days to check the implementation of the final comments and transmits the final CHMP opinion and the product information to the EC and the Member States.
While the linguistic phase is governed by very clear steps and timelines, the transparency and predictability of the decision-making phase – which converts the CHMP opinion into a legal EC decision – could be improved.
Its various steps are less clearly described in publicly available documents, while reporting on actual timelines is less detailed. Actual timelines vary considerably (see below), and no tracking is available of a product’s progress through each step during this phase.
There are four key milestones in the decision-making phase :
- Day 225: Within 15 days of its adoption, the EMA will send the English version of the final CHMP opinion to the Directorate General for Health and Food Safety (DG SANTE), the Member States and the manufacturer, together with a report describing the assessment of the medicinal product by the Committee and stating the reasons for its conclusions. Since all the decisions of DG SANTE must reflect the position of the entire EC as a collegiate body, DG SANTE consults the other services of the EC on the draft decision during a five-day interservice consultation before it is sent to Member States. It is unclear when this starts, and which services are involved in this step.
- Day 240: Within 15 calendar days of receipt of a complete and compliant opinion from the EMA, DG SANTE shares the initial draft of the EC Decision with Member States, who are represented in the Standing Committee on Medicinal Products for Human Use (the “Standing Committee”).
- Day 262: Member States then have 22 calendar days to inform the EC whether they approve the draft, reject it, or wish to abstain from voting. If a decision must be taken urgently, a shorter time limit (usually a minimum of five days) may be set by the EC.
- Day 277: If the Standing Committee votes favorably on the draft decision, the Commission will proceed to the adoption of the decision. The Commission will take a final decision within 15 calendar days following the end of the Standing Committee phase.
Faster approvals save lives
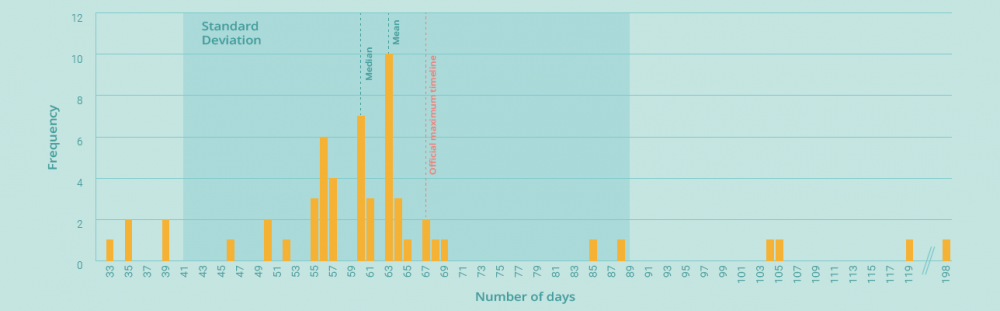
With a maximum official duration of 67 days (and a duration of up to 198 days in practice), the linguistic and decision-making phases described above offer a significant opportunity for improvement – and a real chance to reduce the time it takes for oncology patients to receive the new treatments they need.
An illustrative analysis [see also blog 1] of 11 recently authorized oncology treatments shows that the regulatory steps between CHMP opinion and EC decision together accounted for 18,600 years of potential life lost. Indeed, the full extent of life years lost to delays in approvals is far greater when considering all oncology indications.
While this administrative process only represents a relatively small part of the journey from laboratory to patient, any reduction in the time it takes could have a significant impact on patients and wider society.
A call to concerted action
Vintura, together with a group of more than 30 organizations representing all relevant stakeholders in the European healthcare sector, has established a clear and common understanding of all the steps involved in this 67-day process, and of potential opportunities to shorten timelines.
This work was commissioned by the EFPIA Oncology Platform, and includes several concrete, strategic solutions to shorten the process between CHMP solution and EC decision. The potential impact and feasibility of each solution was considered in order to prioritize quick wins and maximize impact.
Potential solutions – which could be used in combination – include:
- Conducting the decision-making phase in parallel to the linguistic phase, using the English version of the submitted material as a base. This would allow Marketing Authorization to be granted 12 days earlier.
- Increasing the use of digital tools during the linguistic phase, to shorten the phase by 10 days.
- Shortening the written procedure when Member States foresee no objections, to shorten the decision-making phase by 15 days.
These recommended solutions serve as a basis for further dialogue and joint action between stakeholders. Because for cancer patients, Every Day Counts.
Let’s discuss
Inspired to share your thoughts? Or would you like to learn more on how to work together effectively with multiple stakeholders to shorten times to patient access? We would be delighted to hear from you. Please feel invited to contact Bas Amesz.
DO YOU WANT TO READ MORE?
Here are all the articles in the “Every day counts” series
- White paper release: Every day counts – Improving time to patient access to innovative oncology therapies in Europe
- Blog: Bringing stakeholders together to improve patient access to oncology therapies in Europe
- Blog: European access discussions shouldn’t stop at reimbursement
- Blog: Deep dive: The Patient Access Indicator
- Blog: The 10 key factors delaying patient access across Europe
- Blog: Introducing new cancer treatments: how reducing time to Market Access dramatically impacts patients’ lives
- Blog: Speeding up patient access requires dealing with uncertainty on clinical value first, negotiating price second
- Blog: Outdated clinical guidelines prevent timely adoption of oncology innovations into medical practice
- Blog: Europe’s patchwork of evidence requirements is an important factor in delayed patient access
- Blog: Bringing new cancer treatments to Europe: the challenge of making it across all three access milestones
- White paper release: Every day counts –The impact of COVID-19 on patient access to cancer care in Europe.
- Blog: How COVID-19 has affected European patients’ access to cancer care, and what we can learn from the crisis
- Blog: COVID-19 has hurt our ability to reduce cancer deaths
- Blog: 6 learnings from the COVID-19 crisis to make cancer care delivery in Europe more resilient to future challenges
- White paper release: Every day counts – Improving regulatory timelines to optimise patient access to innovative oncology therapies in Europe



