analysing access beyond market access
On a country to country basis, access has been assessed solely along the dimensions of rate of Market Access and time to Market Access: the proportion of oncology therapies with a European marketing authorization that subsequently receive a positive reimbursement decision and delays in reimbursement. Although an essential indicator, patient access after reimbursement traditionally has not been part of the access discussion. Due to its complexity, no formal analysis of actual patient use has been available, leaving the field with an information gap as well as a flawed perspective on access.
Patient access left out of European access discussion
A necessary first step is provided by Vintura’s Patient Access Indicator: a benchmark study to identify European differences in the use of new cancer treatments 12 months after reimbursement. The study puts numbers to what many intuited: a year after reimbursement, patient access varies significantly between countries. Combined with data on delays in time to Market Access, these outcomes provide a new, more complete and more accurate view of access across Europe, as is shown in the graph below. In short, the outcomes indicate that, without exception, all European countries are facing considerable delays during the reimbursement process, delays in actual prescription and use – or both. None are offering their cancer patients optimal access to the latest standard of care and treatment, causing a tremendous impact on individual patients, their families and society.
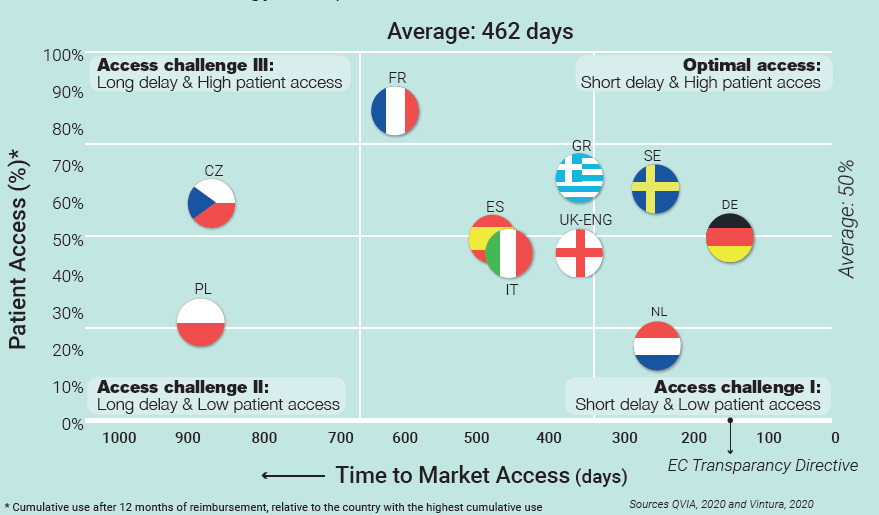
Including patient access to the narrative
Access challenges call for a multi-stakeholder perspective on challenges and solutions, as well as a collaborative approach. For stakeholders, it’s important to realise that introducing a new therapy doesn’t stop at its reimbursement. Measuring the effectiveness of new therapies in terms of rate of Market Access and time to Market Access, as well patient access after reimbursement is vital. After all, given the huge impact on human lives, what value lies in bringing new therapies to market if patients don’t actually get to use those therapies shortly after reimbursement?
Essential: an integral approach
Currently, however, none of the individual stakeholders is responsible for both Market Access and patient access. This is a reflection of the way organisations are structured. Most pharmaceutical companies have a Market Access department focussing solely on reimbursement. Other departments, like Medical and Marketing, are involved only shortly before the launch of a new treatment. In order to address access challenges, something needs to change. In Vintura’s view, an integral approach is needed: from the start, companies should set up a cross-functional team, mandated to ensure both Market Access and patient access. On government side, the industry should be met by authorities that share this scope and mandate. Because only when responsibilities are clearly assigned, a true breakthrough can be achieved in reducing patient waiting times.
LET’S DISCUSS
Inspired to share your thoughts? Or would you like to learn more on how to accelerate patient access? We would be delighted to hear from you. Please feel invited to contact Bas Amesz.
DO YOU WANT TO READ MORE?
Please also be invited to read our others articles in this “Every day counts” series:
- White paper release: Every day counts
- Blog: Bringing stakeholders together to improve patient access to oncology therapies in Europe
- Blog: European access discussions shouldn’t stop at reimbursement
- Blog: Deep dive: The Patient Access Indicator
- Blog: The 10 key factors delaying patient access across Europe
- Blog: Introducing new cancer treatments: how reducing time to Market Access dramatically impacts patients’ lives
- Blog: Speeding up patient access requires dealing with uncertainty on clinical value first, negotiating price second
- Blog: Outdated clinical guidelines prevent timely adoption of oncology innovations into medical practice
- Blog: Europe’s patchwork of evidence requirements is an important factor in delayed patient access



