No formal analysis available
In oncology, it was anecdotally known to all stakeholders: after reimbursement, the implementation speed and level of actual use varies significantly from country to country. Just how big the inequalities between European countries were, we didn’t know: due to its complexity, a formal analysis of this dimension of patient access had never been conducted, leaving the field with an immense information gap.
Filling the information gap on patient access
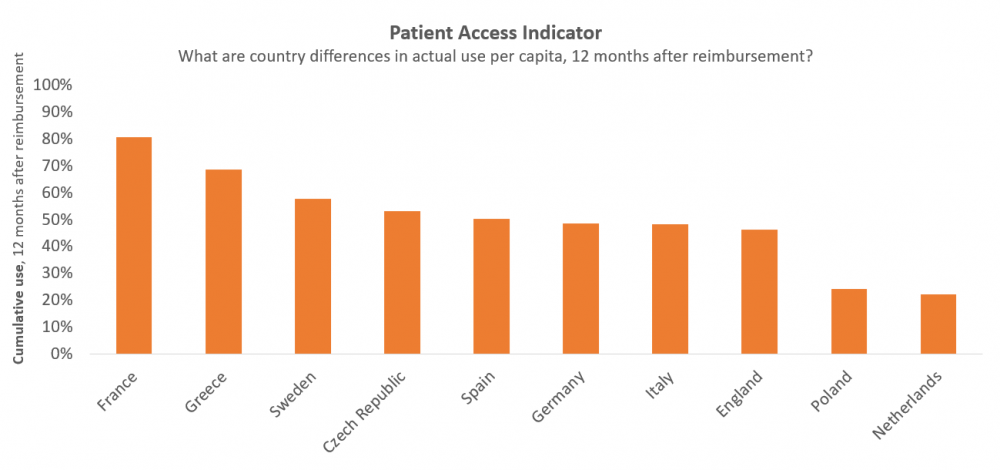
Yet, what value lies in innovation if new therapies don’t find their way to patients? The first of its kind, Vintura’s Patient Access Indicator provides a European benchmark study to identify European differences in the use of new cancer treatments 12 months after reimbursement. We found that countries that perform well on time to Market Access do not necessarily perform well on patient access. This is the case in Germany and The Netherlands. We also found the opposite, for example for France. Read the specifics of our methodology in the full report.
Research methodology: dealing with complexity
For this benchmark, ‘use’ was measured by analysing sales volume per month per capita, using routinely collected business information from pharmaceutical companies and data providers. ‘Post-reimbursement’ was defined as the phase that starts when the first patient is treated under a formal reimbursement scheme. 10 European countries were included as well as 13 oncology therapies, covering 8 cancer types. Per therapy, the cumulative use at 12 months post-reimbursement was expressed as a relative use, compared to the country with the highest use of that therapy. And per country, the average relative use across all therapies was calculated to arrive at one single indicator of post-reimbursement use compared to other countries.
The outcomes may also serve as a reminder that patient access doesn’t stop at reimbursement, making it not just the Market Access department’s business.
Next step: understanding the differences
In European countries facing delays in patient access, 4 important factors play a role: multiple layers of decision-making, insufficient budget to implement decisions, suboptimal healthcare structure and a low frequency of clinical guideline updates. A benchmark, The Patient Access Indicator illustrates differences between countries rather than explanations. However, since the study covers multiple therapies in multiple indications, it provides a good indication of health system factors posing a barrier to patient access. Combined with the aforementioned factors, the outcomes of the Patient Access Indicator may serve as input for national discussions aimed at identifying the key factors underlying a relatively low level of patient access. The outcomes may also serve as a reminder that patient access doesn’t stop at reimbursement, making it not just the Market Access department’s business.
Discover why European access discussions shouldn’t stop at reimbursement.
LET’S DISCUSS
Inspired to share your thoughts? Or would you like to learn more on how to accelerate patient access? We would be delighted to hear from you. Please feel invited to contact Bas Amesz.
DO YOU WANT TO READ MORE?
Please also be invited to read our others articles in this “Every day counts” series:
- White paper release: Every day counts
- Blog: Bringing stakeholders together to improve patient access to oncology therapies in Europe
- Blog: European access discussions shouldn’t stop at reimbursement
- Blog: Deep dive: The Patient Access Indicator
- Blog: The 10 key factors delaying patient access across Europe
- Blog: Introducing new cancer treatments: how reducing time to Market Access dramatically impacts patients’ lives
- Blog: Speeding up patient access requires dealing with uncertainty on clinical value first, negotiating price second
- Blog: Outdated clinical guidelines prevent timely adoption of oncology innovations into medical practice
- Blog: Europe’s patchwork of evidence requirements is an important factor in delayed patient access



